|

New
labeling rules in effect
April 1999
On
March 23, 1999 new rules went into effect for labeling of dietary
supplement products including a new information panel titled "Supplement
Facts". Products labeled prior to March 23rd can continue to be
sold until stocks are depleted.
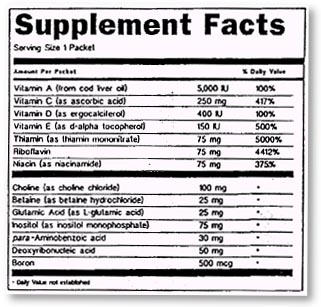
The
"Supplement Facts" panel must include specific information about
ingredients in the product. It is similar in format to the "Nutrition
Facts" panel that appears on most processed foods.

A
new dosage recommendation standard called DV (daily values) must
be included in the Supplement Facts panel for ingredients where
a DV has been established. DVs are based on DRIs (dietary reference
intakes). "DRI" is an umbrella term for groups of values -- including
RDAs, AIs, EARs, and UIs -- that specify recommended dosages proposed
by the Food and Nutrition Board of the Institute of Medicine of
the National Academy of Sciences in partnership with Health Canada.
This is a departure from the U.S. RDAs used on food labeling in
that DRIs attempt to incorporate new information about nutrition
needed to prevent disease.

The
new rules are as follows:
A
statement of identity must appear on the front panel of the product
label. The statement must use the terms "dietary supplement" or
a term identifying the contents of the product, such as "Vitamin
C supplement" or "Herbal supplement".
All
ingredients in the product must be declared in the ingredient statement
or within the "Supplement Facts" panel.
The
"Supplement Facts" panel must show the following information:
-
The manufacturer's suggested serving size for consumption at
one occasion. All ingredient information must be based on this
serving size.
-
Information on nutrients when they are present in significant
levels, such as vitamins A and C, calcium, iron, and sodium,
and the percent Daily Value recommendation for consumption where
a reference has been established.
-
All other dietary ingredients present in the product, including
botanicals and amino acids, for which no Daily Value has been
established. They must be listed below those with a DV separated
by a bar. The quantity present in each ingredient must be listed
and they must be identified as having no DV.
-
Herbal products must be identified by the common or usual name
and the part of the plant used to make the supplement (such
as root, stem or leaf). If the common or usual name is not listed
in Herbs for Commerce published by the American
Herbal Products Association (see description),
the Latin binomial name such as Tercoma mollis.HBK or Cecropia
obstusifolia Bert. must be listed.
-
Proprietary blends may be listed with the weights given for
the total blend only. When this is done, components of the blend
must be listed in descending order of predominance by weight.
The
rule also specifies a minimum type size and flexible formats.
When
the terms "high potency" and "antioxidant" appear on the label of
a dietary supplement, the following applies:
-
"High
potency" may be used to describe a nutrient when it is present
at 100% or more of the RDA established for that vitamin or mineral.
"High potency" may also be used with multi-ingredient products
if two-thirds of the nutrients that are in the product are present
at levels that are more than 100% of the RDA.
-
"Antioxidant"
may be used in conjunction with currently defined claims for
"good source" and "high" to describe a nutrient where scientific
evidence shows that following absorption of a sufficient quantity,
the nutrient (such as vitamin C) will inactivate free radicals
or prevent free radical-initiated chemical reactions in the
body.
In
addition, the label must include dosage directions, the net quantity
of the contents (eg, 100 tablets), and the name and place of business
of the manufacturer, packer, or distributor.


|