|
|
|
|

-
What's in Your Multi-vitamin?
- Doctors
now recommend taking a daily multi-vitamin, and millions of Americans
are doing so—but how do you choose among the hundreds of
brands and formulations on the market? (June 2004)
-
-
Vitamins - Recommended daily intakes
- Chart
of recommended minimums and maximums. (June 2004)
-
-
Minerals - Recommended daily intakes
- Chart
of recommended minimums and maximums. (June 2004)
-
-
When "Fatty" is Good: Omega-3 Oils and Fatty Acids"
- Many
Americans have embraced low-fat diets and foods. But some fats
help us fight heart disease, diabetes, and cancer. What are these
"good" fats and how do we get enough of them? (May 2004)
-
-
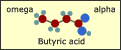 Chemical
and Physical Structure of Fatty Acids Chemical
and Physical Structure of Fatty Acids
- The
terminology surrounding fatty acids can be confusing. Looking
at their structure helps us understand which are the "good fats"
and which are the "bad" ones. (May 2004)
-
-
Trade Organizations Support Actions Banning "Andro"
- Two
trade organizations announce support of sports organizations banning
athletes' use of steroid precursors and FDA's action on andro.
They also reaffirm support for Biden-Hatch legislation. (March
2004)
-
-
Harvard Medical School Publishes Buyer's Guide to Herbs and Supplements
- Guide
covers 52 herbs and supplements, including possible side effects,
interactions, safety precautions, and dosage. Each supplement
gets a report card on its effectiveness for various conditions.
(January 2004)
-
-
Novogen Gains Health Supplement Patent
- Novogen
Limited has been granted the U.S. patent covering health supplements
based on the use of soy isoflavones in tablet or capsule form.
(December 2003)
-
-
FDA Stranging Consumer Health
- The
high cost of prescription drugs poses serious challenges for American
families and city and state budgets. Should we import cheaper
drugs from abroad—and are there better alternatives? (November
2003)
-
-
Blue Cross/Blue Shield Says 1.1 Million Teens Have Used Performance-Enhancing
Sports Supplements And Drugs
- Blue
Cross/Blue Shield calls for increased educational efforts among
parents and teams (October 2003)
-
-
Supplement Industry Associations Support Biden/Hatch Legislation
Placing 'Andro' Under Controlled Substances Act
- Bill
would remove steroid hormone precursors from the dietary supplement
marketplace. Continued controversy on this topic is damaging the
industry, the agency, and athletes who could be banned for using
andro and similar substances. (October 2003)
-
-
California bans supplements with ephedra
- Legislation
becomes effective 1 January 2004. (October 2003)
-
-
Judge Denies Class-Action Certification On Ephedra Lawsuit
- A
federal judge in Miami ruled that a lawsuit filed by six individuals
concerning Metabolife 356 did not meet at least three federal
requirements for being granted class-action status. (October 2003)
-
-
Proposed GMPs Seen as Costlier Than FDA Estimates
- The
American Herbal Products Association asserts FDA's proposed GMPs
will cost more than 800% of the FDA estimate and sees economic
benefits of only 10% of FDA estimate. (September 2003)
-
-
FDA's New Industry Regulations Contain Troubling Flaws
- New
FDA regulations will require testing designed to ensure that bottle
contents match labels. This will improve quality, but the regulations
have serious flaws that consumers will pay for through unnecessarily
increased prices. (August 2003)
-
-
DSQI Launches Unique Quality Survey
- Survey
allows consumers to rate their experience with the quality of
specific brands of supplements, both good and bad. Registered
participants can also see aggregated data on consumer preferences
and comments on individual products. Despite its anecdotal nature,
survey information will help distinguish which companies and products
represent superior value. (August 2003)
-
-
Council for Responsible Nutrition Says Lancet Researcher Conclusions
Are Irresponsible
- CRN
says conclusions of meta-study of vitamin E and beta-carotene
published in Lancet are: irresponsible, overinterpreted,
and old news disguised as new for publicity purposes. (June 2003)
-
-
Illinois Bans Sale of Ephedra
- Selling
ephedra in Illinois is now a misdemeanor, punishable by up to
1 year in jail and $5000 fine. (May 2003)
-
-
Juvenon Initiates Cardiovascular Disease Clinical Trial
- Juvenon
is launching an NIH-funded clinical trial, to be conducted at
the Boston University School of Medicine, to evaluate the effect
of Juvenon Energy Formula on endothelial function in elderly patients
with coronary artery disease. (May 2003)
-
-
Misbranded Dietary Supplements Destroyed
- FDA
announced that Nature's Youth has completed its voluntary destruction
of the misbranded product "Nature's Youth hGH" and will change
its product labeling to comply with the law. (May 2003)
-
-
Industry Advises Against Use of Dietary Supplements As Remedy
for SARS
- Industry
groups recommend that consumers NOT use dietary supplements to
treat or prevent SARS, and that retailers not stock any products
claiming to do so. (April 2003)
-
-
New Labeling Program Delivers Safety Information for Herbal Products
- American
Botanical Council's new Safety Labeling Program helps manufacturers
put science-based safety information on herbal supplement labels.
(March 2003)
-
-
American Botanical Council Reviews Ephedra Safety
- ABC
monograph on ephedra safety details recommended dosage levels,
side effects and contraindications, also describes how ephedra
affects the body. (March 2003)
-
-
DHHS and FDA Release RAND Report on Ephedra and Propose New Warnings
- RAND
review of 16,000 adverse events on ephedra finds 21 'sentinel
events', while the FDA and HHS propose specific warning labels.
(February 2003)
-
-
AMA Applauds FDA Action to Protect Public From Dangers of Ephedra
- AMA
calls ephedra a serious threat to public health and applauds HHS
Secretary Thompson and FDA Commissioner McClellan for actions
to protect public through warning labels. (February 2003)
-
-
Hyperhealth CD-ROM Contains 20,000 Pages Of Information About
Supplements, Health And Disease
- Hyperhealth
CD-ROM is touted as an easy-to-use encyclopedia with thousands
of topics ranging from health strategies to herbs, foods and nutritional
supplements. (February 2003)
-
-
FDA Pursues Better Health Information For Consumers
- The
FDA is encouraging supplement manufacturers to make accurate,
science-based claims about product health benefits and simultaneously
cracking down on companies making bogus marketing claims. (December
2002)
-
-
CRN Supports Government Efforts Toward Better Health Information
And Enforcing Regulations
- The
Council For Responsible Nutrition commends FDA on combating misleading
advertising and on recognizing qualified health claims for conventional
foods and dietary supplements. (December 2002)
-
-
Harris Poll Shows Widespread Public Ignorance Of Supplement Regulation
- Many
Americans are misinformed about how supplements are regulated
by the government. (December 2002)
-
-
Military Bans Sales of Ephedra Supplements
- All
ephedra products are now banned from stores on Army and Air Force
bases, as well as the Navy and Marine Corps. (November 2002)
-
-
CRN Issues Guidelines for Young Athletes on Responsible Use Of
Sports Nutrition Supplements
- CRN
guidelines categorize supplements based on knowledge about suitability
and/or dangers for young athletes. (November 2002)
-
-
Industry Supports Strong Warning Label For Products With Ephedra
- Industry
supports stringent and mandatory warnings on products that contain
ephedra. Such warnings have already been adopted by virtually
all manufacturers. (October 2002)
-
-
IOM Report on Macronutrients Misses the Mark on Omega-3 Fatty
Acids
- Levels
of omega-3 fatty acids recommended by the national Institute of
Medicine are much lower than those found beneficial in reducing
the risk of heart disease. (September 2002)
-
-
Government Launches Criminal Investigation Of Metabolife
- The
FDA will examine 13,000 phone calls to Metabolife hotline for
evidence of ephedra-related risks. The Department of Justice is
investigating if Metabolife lied to the FDA about health-related
customer complaints. (August 2002)
-
-
New Law Limits Use of Term "Ginseng"
- All
products called ginseng must now be derived from Panax ginseng.
(August 2002)
-
-
Government Will Study Ephedra Risks
- Following
a comprehensive review by RAND Corporation of ephedra research,
the National Institutes of Health will design and perform a new
study of ephedra safety risks. (June 2002)
-
-
Speak Your Mind On Supplement Regulation
- International
regulations set by Codex could become law in the US. The US government
is seeking public comments by July 30 on what policies to adopt
for the November meeting in Berlin. (June 2002)
-
-
Sports Supplements: What Are The Risks?
- What
are the benefits and risks of performance enhancing supplements?
The MayoClinic.com website offers valuable insights for weekend
warriors and professional athletes alike. (June 2002)
-
- FDA
Seeks Public Comment on First Amendment Limits on Health Claims
- The
FDA recently lost another court decision regarding the way it
restricts health claims, and now seeks public comments about its
approach to health claims for foods, drugs, and dietary supplements.
(May 2002)
-
-
Supplement Makers Busted
- State
and federal agents seized assets of company that marketed supplements
for penis and breast enlargement. (May 2002)
-
- Calcium
Counter Makes It Easy to Calculate Daily Intake
- New
calcium counter measures intake with just nine simple but important
questions -- for men and women of all ages. (May 2002)
-
- Cancer
Scientists Find Prescription Drugs In Herbal Supplement
- PC
SPES is an effective herbal remedy against prostate cancer. However,
cancer researchers report finding three prescription drugs in
samples they analyzed. Supplements are not allowed to contain
drugs. (April 2002)
-
- Toxicologist
Reviews Kava Cases Linked to Liver Disease
- Toxicologist
says medical evidence does not show kava supplements caused liver
toxicity. (March 2002)
-
- Kava
May Be Linked To Liver Problems
- Twenty-nine
cases of serious liver disease in Europe among patients using
high levels of kava have sparked government action and prompted
a toxicology review. (February 2002)
-
- Two
Industry Leaders Form Quality Certification Alliance
- NSF
International and the National Nutritional Foods Association form
alliance giving all dietary supplement companies access to third-party
testing, manufacturing inspections, and product certification.
(December 2001)
-
-
- Supplements
and Surgery - What to Tell Your Doctors
- This
article gives patients a solid foundation for discussing herbs
and surgery with their doctors. (September 2001)
-
- Vitamin
C and DNA Damage: Real Danger or False Alarm?
- Vitamin
C again appears in headline warnings that it could cause DNA damage
-- but this "news" is based on a single study, not wide-ranging
research that would raise real caution flags. (July 2001)
-
- Mad
Cow Disease and Supplements
- Are
Americans at risk from mad cow disease in dietary supplements?
We investigated both the risk and government action being taken
to protect public health. (April 2001)
-
- Scientists
To Debate RDAs
- Next
spring's Experimental Biology 2002 conference will include a special
session that debates the adequacy of several of the new recommended
dietary allowances (RDAs). (April 2001)
-
- RDAs
And Safe Upper Levels: Solid Science Versus Bureaucratic Bias
- Experimental
data for the new RDAs and safe upper levels are sketchy, and at
least one authority claims that RDAs and safe upper levels are
more political than scientific. (March 2001)
-
- Enter
Supplement Quality, Stage Right . . .
- New
developments in testing and standards programs will, in the long
run, ensure that consumers can purchase dietary supplements with
confidence that products are well-made, safe, effective, and contain
the ingredients and potencies listed on the label. (December 2000)
-
- Ephedra
Controversy Heats Up Again
- Ephedra
is a controversial ingredient in many popular herbal weight-loss
products. We report on FDA claims that ephedra causes injuries
and deaths, and on industry claims that ephedra is safe for healthy
people when used as directed. (September 2000)
-
- Blurry
Line Between Supplements and Drugs
- A
recent court ruling bans the use of a valuable dietary supplement
because of an obscure section of DSHEA that was designed to protect
drug producers. (August 2000)
-
- Court
Finds FDA Rejection of Truthful Health Claims Unconstitutional
-
A U.S. Court of Appeals upheld the consumer's ability to interpret
health claims on labels when properly disclaimed. (January 1999)
-
NNFA Puts Teeth into its Quality
Standards Compliance Program -
The National Nutritional Foods Association (NNFA) has launched
three new quality programs. (October 1998)
-
-
See also Letters to
the Editor.
 |
 |
| |
|
|
|